There's been a big story in the UK this week about the clinical trial of a drug called TGN1412, produced by TeGenero, which provoked a response in the test subjects that caused intense pain and multiple organ failure. TGN1412 is described as a "humanised agonistic anti-CD28 monoclonal antibody", meaning that it's an antibody that appears human—so as not to be immediately cleared from the body by the immune system—and which stimulates the cell surface receptor CD28. The strange thing is that, in these trials, the antibody seems to have done exactly what you'd expect it to do.
Some background: Antigen-presenting cells (APCs) pick up antigens (generally proteins), break them into small pieces and present them on their surface attached to MHC molecules (see the figure below). If the APC has encountered signals of pathogenicity such as bacterial surface proteins or responses to viral attack while it was picking up the antigen, it will have costimulatory molecules CD80 and CD86 on its surface. When a T cell binds to those small pieces (peptides) of antigen on the surface of an APC, using its T cell receptors (TCRs), it is activated, but in the absence of costimulatory signals (in other words, when the antigen being presented is most likely a protein from a harmless self cell, or a commensal bacteria, or some food or dust) it is only partially activated and ends up either tolerised or deleted (so it can't respond to that antigen in the future) or possibly becomes a regulatory T cell which can suppress the responses of other T cells to that same antigen. If CD80 or CD86 are present on the APC, however, these bind to CD28 on the surface of the T cell which is then fully activated, proliferates and produces an effective response to attack and eliminate the pathogen from which the antigen was derived.
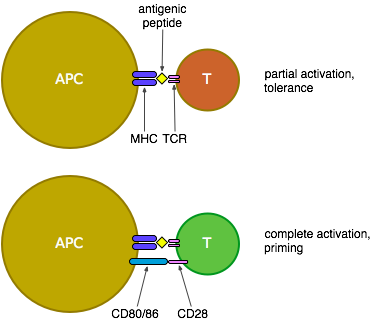
An agonist to CD28 would be expected to stimulate T cells in collaboration with antigen recognition through the TCR. If this isn't targeted to T cells of any particular specificity, it could well activate immune responses against both self- and tumour-derived antigens.
In contrast, superagonists of CD28, which are stronger and activate the T cells even without any TCR signal, have also been shown to cause T cell proliferation but are said to result in a larger pool of anti-inflammatory IL-10-producing regulatory T cells (which could suppress future immune responses) than activated T cells (depending on the dose of antibody used). This is the kind of effect that could be useful against inflammatory conditions like rheumatoid arthritis.
- Superagonistic anti-CD28 antibodies: potent activators of regulatory T cells for the therapy of autoimmune diseases.
- Transient accumulation of human mature thymocytes and regulatory T cells with CD28 superagonist in "human immune system" Rag2-/-{gamma}c-/- mice.
- Selective targeting of regulatory T cells with CD28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis.
- Efficient expansion of regulatory T cells in vitro and in vivo with a CD28 superagonist.
- CD28 superagonists: mode of action and therapeutic potential.
- Treatment and prevention of experimental autoimmune neuritis with superagonistic CD28-specific monoclonal antibodies.
- Topological requirements and signaling properties of T cell-activating, anti-CD28 antibody superagonists.
- Effective treatment of adjuvant arthritis with a stimulatory CD28-specific monoclonal antibody.
TeGenero had suggested that TGN1412, a CD28 superagonist, would be useful for treating both a) inflammatory conditions like rheumatoid arthritis, in which the immune system is overactive and needs to be suppressed and b) B-cell chronic lymphocytic leukemia, in which the immune system needs to be activated in order to attack and eliminate the malignant B cells.
On the TeGenero website, TGN1412 is known as CD28-SuperMAB. As mentioned in a story in The Times, TGN1412/CD28-SuperMAB is similar to the superagonistic antibody JJ316 used in many of the papers listed above (most of which were co-authored by Thomas Hanke and Thomas Hünig who created/discovered TGN1412, both of whom have a
I can only speculate, as I don't know the details of the experiments and it could have been a problem with the manufacture or administration of the antibody that caused the large adverse reaction in the test subjects, but it does seem like TeGenero are making paradoxical immune-stimulating and anti-inflammatory claims for their drug that haven't been well demonstrated so far. There must be some convincing evidence of the drug's effectiveness though, as otherwise it wouldn't have passed the regulatory process that allows clinical trials to take place.
If it does turn out that the earlier animal trials showed the drug to be effective and safe, then there's a much stronger case against the relevance of research on animals for the development of human treatments. The earlier experiments that TeGenero carried out in animals included rats, rabbits and monkeys, though the details of the later experiments aren't publically available. It seems that the primate experiment may have been simply an ex vivo treatment of cells with TGN1412, which were then injected into 1 rhesus monkey - no side effects were observed. In one paper describing administration of JJ316 to rats, "therapeutic regimens over a broad dose range were never accompanied by detectable side effects. Thus, both the pharmacodynamics and the toxicological profile of CD28 superagonists support broad applicability of this class of antibody in humans."